Research topics
Axe 1
Fate of nanoparticles and metals in humans
Axe 4
Extracellular Vesicles : production, engineering and characterization

Axe 2
Nanomedicine approaches to modulate the microenvironment of solid tumors
Axe 5
Characterizing the intercellular transport mediated by extracellular vesicles (exosomes)
Axe 3
Extracellular Vesicles and Regenerative Medicine
Axe 6
Embryo models and regenerative medecine
Axe 1: Fate of nanoparticles and metals in humans
Humans are increasingly exposed to nanoparticles (NPs), which originate from various manufactured products, such as pesticides, food, textiles, cosmetics or paints, that are made by combustion or vehicle emissions, or that are introduced in the body for medical purposes as the nanomedicines we develop in the lab. This growing exposure to NPs questions their intimate interactions with biological components and their impact on human health. Since two decades, our group investigates how nanoparticles with different compositions, organization, shape and surface state are processed by cells and how they can be metabolized, sequestered or eliminated by the organism. Following a fairly different approach from toxicologists, we investigate how nanoparticles can be reshaped, transformed, degraded or even recycled by the biological environments at short and long terms, with a material science and biophysical point of view. We also study the mechanisms by which NP or their constituants can be disseminated and accumulated in the organism. As we develop theranostic applications of photo- and magnetically-activable nanoparticles, we are particularly interested into magnetic iron-oxide nanoparticles, carbon nanotubes, gold and silver nanoparticles, and other metallic nanostructures.
Press release
http://www.cnrs.fr/fr/que-deviennent-les-nanoparticules-dor-dans-les-cellules
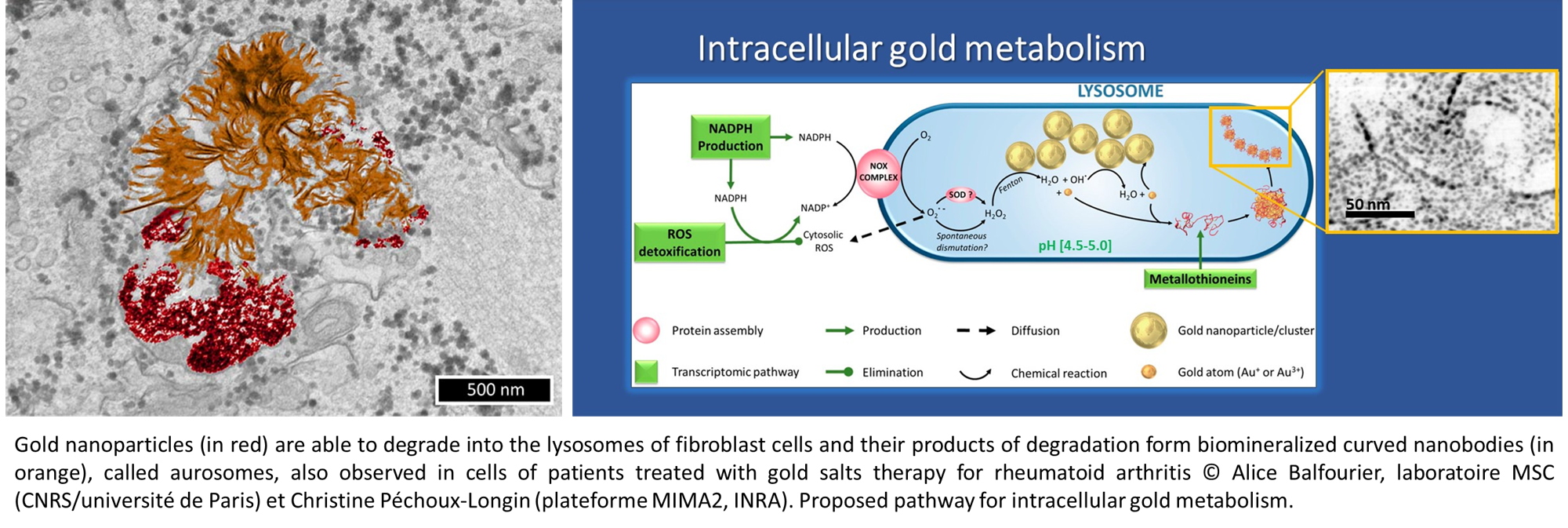
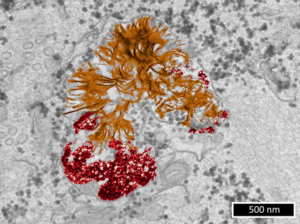
Gold degradation
While gold nanoparticles are at the core of an increasing range of medical applications, their fate in the organism has barely been studied so far. Because of their chemical inertness, common belief is that gold nanoparticles remain endlessly intact in tissues.We show that 4- to 22-nm gold nanoparticles are actually degraded in vitro by cells, with a faster degradation of the smallest size. Transcriptomics studies reveal the active role of cell lysosome into this biodissolution. Furthermore, we point out that the released gold recrystallizes into biopersistent nanostructures. Interestingly, these degradation products are similar to previously observed gold deposits in human tissues after gold salts treatment for rheumatoid arthritis, underlying a common metabolism between gold nanoparticles and ionic gold.
Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. A Balfourier, N Luciani, G Wang, G Lelong, O Ersen, A Khelfa, D Alloyeau, F Gazeau*, F Carn* PNAS, 2020, 117 (1) 103-113; https://www.pnas.org/cgi/doi/10.1073/pnas.1911734116
Despite an abundant literature on gold nanoparticles use for biomedicine, only a few of the gold-based nanodevices are currently tested in clinical trials, and none of them are approved by health agencies. Conversely, ionic gold has been used for decades to treat human rheumatoid arthritis and benefits from 70-y hindsight on medical use. With a view to open up new perspectives in gold nanoparticles research and medical use, we revisit here the literature on therapeutic gold salts. We first summarize the literature on gold salt pharmacokinetics, therapeutic effects, adverse reactions, and the present repurposing of these ancient drugs. Owing to these readings, we evidence the existence of a common metabolism of gold nanoparticles and gold ions and propose to use gold salts as a “shortcut” to assess the long-term effects of gold nanoparticles, such as their fate and toxicity, which remain challenging questions nowadays.
Gold-based therapy : from past to present. Balfourier, A, Kolosnjaj-Tabi J, N Luciani, F Carn, F Gazeau,. PNAS 117 (37), 22639-22648 https://doi.org/10.1073/pnas.2007285117
Carbon Nanotubes
Iron Oxide
Axe 2: Nanomedicine approaches to modulate the microenvironment of solid tumors
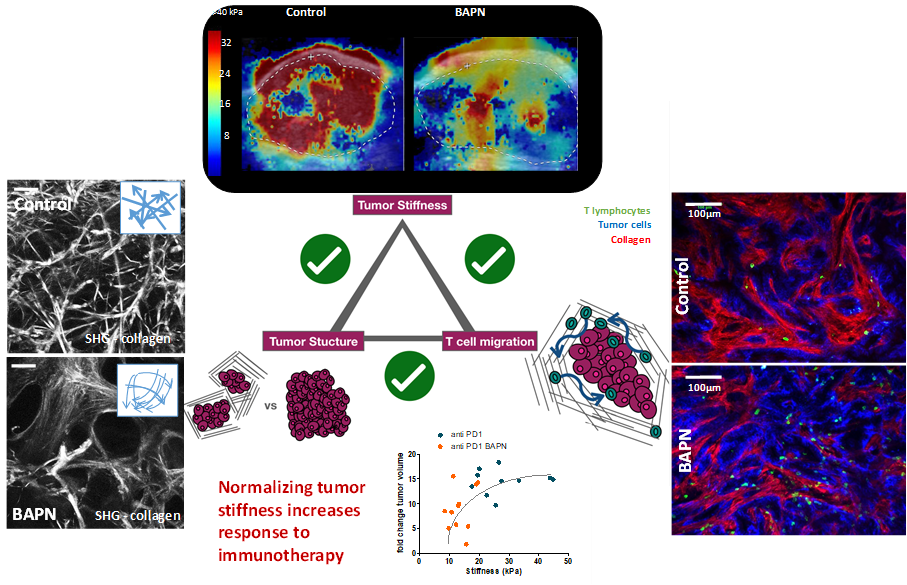
Solid tumors are heterogeneous ever evolving ecosystem comprising not only tumor cells but also a stroma containing fibroblasts, extracellular matrix, immune cells, with aberrant physical properties in comparison to healthy tissue (increased internal pressure, increased stiffness, chaotic vascularisation, hypoxia…). Desmoplastic tumors such as pancreatic ductal adenocarcinoma or cholangiocarcinoma are characterized by a very stiff extracellular matrix which raises a barrier against the penetration of chemotherapy as well as immune cells, which can explain the resistance of such cancer to most current treatment as well as immunotherapeutic approaches. Tackling the tumor microenvironment and normalizing its physical properties is an attractive strategy to make the tumor more sensitive to chemo and immunotherapies. In a recent study, we established the link between tumor architecture, tumor stiffening and the ability of T cell to infiltrate the tumor (elife 2021). We are developping nanomedicine approaches to modulate the tumor microenvironment with a spatial and temporal control owing to the external activation of nanoparticles
How tumor stiffness affect immune cell infiltration?
Solid tumors are heterogeneous ever evolving ecosystem comprising not only tumor cells but also a stroma containing fibroblasts, extracellular matrix, immune cells, with aberrant physical properties in comparison to healthy tissue (increased internal pressure, increased stiffness, chaotic vascularisation, hypoxia…). Desmoplastic tumors such as pancreatic ductal adenocarcinoma or cholangiocarcinoma are characterized by a very stiff extracellular matrix which raises a barrier against the penetration of chemotherapy as well as immune cells, which can explain the resistance of such cancer to most current treatment as well as immunotherapeutic approaches. Tackling the tumor microenvironment and normalizing its physical properties is an attractive strategy to make the tumor more sensitive to chemo and immunotherapies. In a recent study, we established the link between tumor architecture, tumor stiffening and the ability of T cell to infiltrate the tumor (elife 2021). We are developping nanomedicine approaches to modulate the tumor microenvironment with a spatial and temporal control owing to the external activation of nanoparticles.
Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Alba Nicolas-Boluda, Javier Vaquero, Lene Vimeux, Thomas Guilbert, Sarah Barrin, Chahrazade Kantari-Mimoun, Matteo Ponzo, Gilles Renault, Piotr Deptula, Katarzyna Pogoda, Robert Bucki, Ilaria Cascone, José Courty, Laura Fouassier, Florence Gazeau*, Emmanuel Donnadieu*
eLife 2021;10:e58688 doi: 10.7554/eLife.58688
Videos:
Treated with BAPN – https://static-movie-usa.glencoesoftware.com/mp4/10.7554/442/776d6889c8c8397cd70f8490229c2e342d35e7f9/elife-58688-video1.mp4
Nanohyperthermia to reverse stiffness in desmoplastic tumors
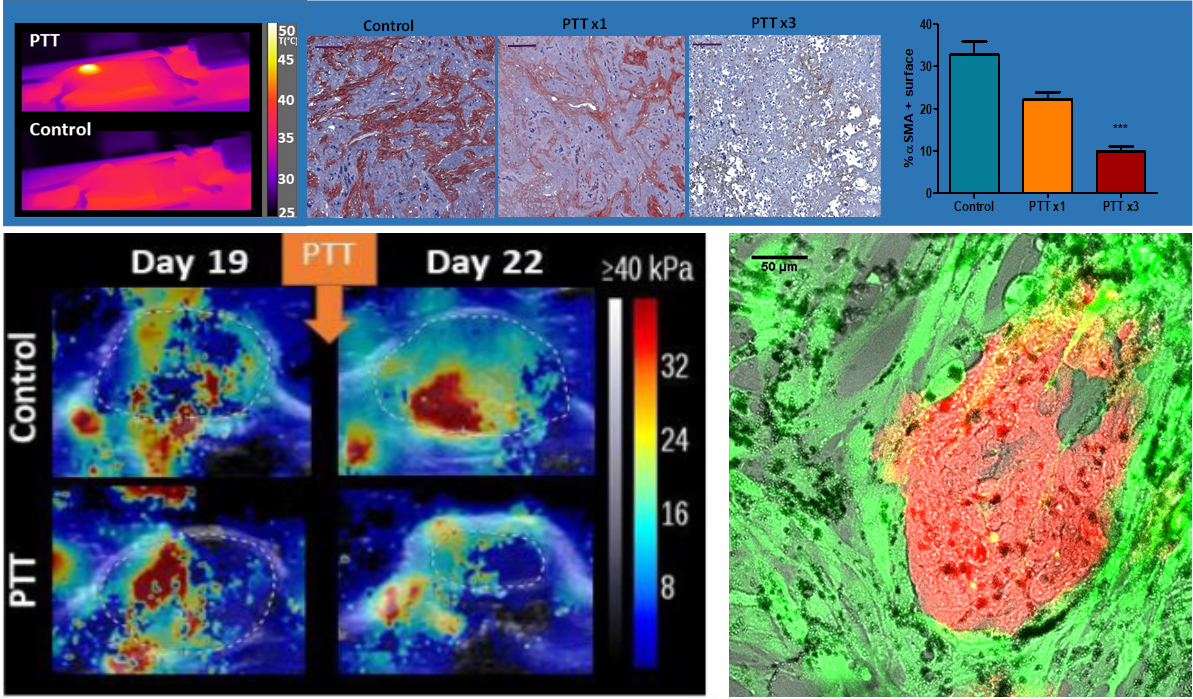
Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. A Nicolás-Boluda, J Vaquero, G. Laurent, G Renault, R Bazzi, E Donnadieu, S Roux, L Fouassier,* F Gazeau*. ACS Nano, 2020, 14, 5, 5738–5753 doi.org/10.1021/acsnano.0c00417
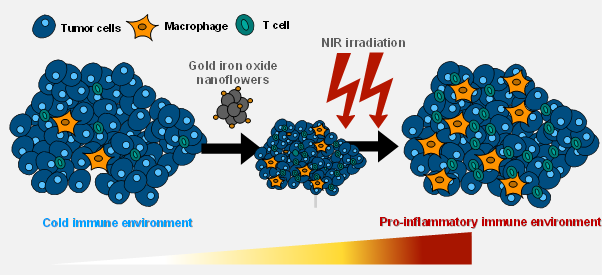
Gold decorated iron oxide Nanohyperthermia modulate tumor immune response

Two step promotion of a hot tumor immune environment by gold decorated iron oxide nanoflowers and light-triggered mild hyperthermia, Alba Nicolas-Boluda, Gautier Laurent, Rana Bazzi, Stéphane Roux, Emmanuel Donnadieu and Florence Gazeau, Nanoscale, 2021, 13, 18483–18497 DOI: 10.1039/d1nr03201a
Nanohyperthermia as an alternative to HIPEC (hyperthermic intraperitoneal chemotherpy) to treat peritoneal metastasis
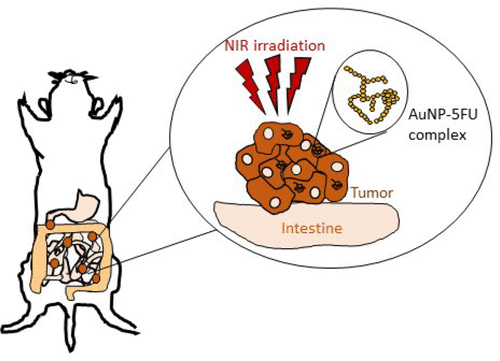
Tumor-Selective Immune Active Mild Hyperthermia Associated with Chemotherapy in Colon Peritoneal Metastasis by Photoactivation of Fluorouracil – Gold Nanoparticles Complexes V Mulens-Arias, A Nicolás-Boluda, A Pinto, A Balfourier, F Carn, A K A Silva, M Pocard, F Gazeau* ACS Nano, February 2, 2021 DOI:10.1021/acsnano.0c10276
Nanohyperthermia : towards intracellular precision
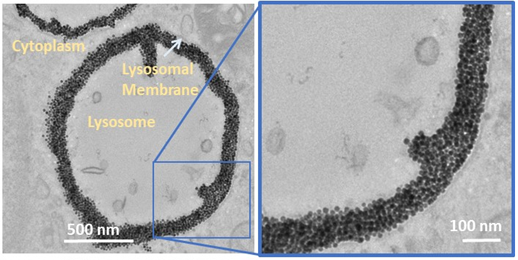
Intracellular Fate of Hydrophobic Nanocrystal Self‐Assemblies in Tumor Cells
A Nicolas‐Boluda, Z Yang, I Dobryden, F Carn, N Winckelmans, C Péchoux, P Bonville, S Bals, P Martin Claesson, F Gazeau*, M P Pileni * Advanced Functional Materials,16 August 2020 https://doi.org/10.1002/adfm.202004274
Self-assemblies of Fe3O4 nanocrystals: towards nanoscale precision of photothermal effects in the tumor microenvironment. A Nicolas-Boluda, Z Yang, T Guilbert, L Fouassier, F Carn, F Gazeau,* MP Pileni*
Advanced functional materials 2020/10/15 2006824 https://doi.org/10.1002/adfm.202006824
Immune Reprogramming Precision Photodynamic Therapy of Peritoneal Metastasis by Scalable Stem Cell Derived Extracellular Vesicles
Immune Reprogramming Precision Photodynamic Therapy of Peritoneal Metastasis by Scalable Stem Cell Derived Extracellular Vesicles » A Pinto, I Marangon, J Méreaux, A Nicolás-Boluda, G Lavieu, C Wilhelm, L Sarda, A K A Silva, M Pocard*, F Gazeau* ACS Nano, January 22, 2021, https://doi.org/10.1021/acsnano.0c09938
Axe 3: Extracellular Vesicles (EVs) and Regenerative Medicine
Our aim is to develop extracellular vesicle (EV)-based therapies with high translational potential for regenerative medicine. We pioneered the concept of turbulence vesiculation for high-yield and large-scale EV production by a turbulent flow stimulation in bioreactors complying with regulatory standards for clinical batch production. We also investigate thermo-controlled EV delivery in stimulus-responsive hydrogels for local administration with a synergic mechanical effect, for instance, for fistula therapy or oesophageal stricture preventions.
Digestive fistulas are a major health burden related to Crohn’s disease or secondary to surgery, cancer therapy or trauma. Digestive fistulas are an abnormal communication between two digestive organs or a digestive organ and the skin representing challenging conditions associated with low remission rates and high refractoriness. There is an urgent need of novel therapeutic approaches for this disease. Such unmet needs in digestive fistula management motivated the investigation of cell therapy based on the local administration of stromal cells (SCs). These cells display multiple therapeutic effects and one of the main ones is to reduce the inflammation favoring the fistula healing process. Although cell therapy results are encouraging, they are not fully satisfactory in terms of percentage of patients that achieve log-term disease remission, leaving place for a second generation therapy. SCs are known to release pro-survival and anti-inflammatory signals by means of extracellular vesicles (EVs) pointing out EV role in regenerative medicine.
We investigate an alternative to cell therapy approach, by proposing a minimally-invasive cell-free local therapy based on the regenerative effect of EVs from SCs. Formerly regarded as a “cell dust”, EVs, specially from SCs, are now considered as regenerative agents able to play an important role in the healing process. Previous studies showed the beneficial effect of SC EVs in the therapy of heart, kidney, liver, brain and skin injuries (Figure 1). We consider that SC EVs represent an eligible alternative for fistula therapy, as they recapitulate the regenerative effect of their mother cells while mitigating risks of uncontrolled replication and differentiation while offering “off-the-shelf”, storage and shelf-life gains. Our previous results in a clinically relevant fistula disease model clearly indicated that a therapy based on SC EVs was able to promote post-surgical digestive fistula healing.
Today, the main challenges for rendering EV-based regenerative medicine clinically feasible are large-scale high-yield standardized EV production and EV optimized administration. Concerning EV manufacturing, stringent requirements must be considered such as up-scaled and high-yield production fulfilling uniformity, consistency, purity and reproducibility criteria based on standardized and reliable quality control and compliance to good manufacturing practices (GMP). The way EVs are administered also represents a main concern considering that systemic administration results in rapid EV clearance and localization in off-target organs.
We have the ambition to render viable the implementation of EV-based therapy by tackling EV production and administration technical barriers.
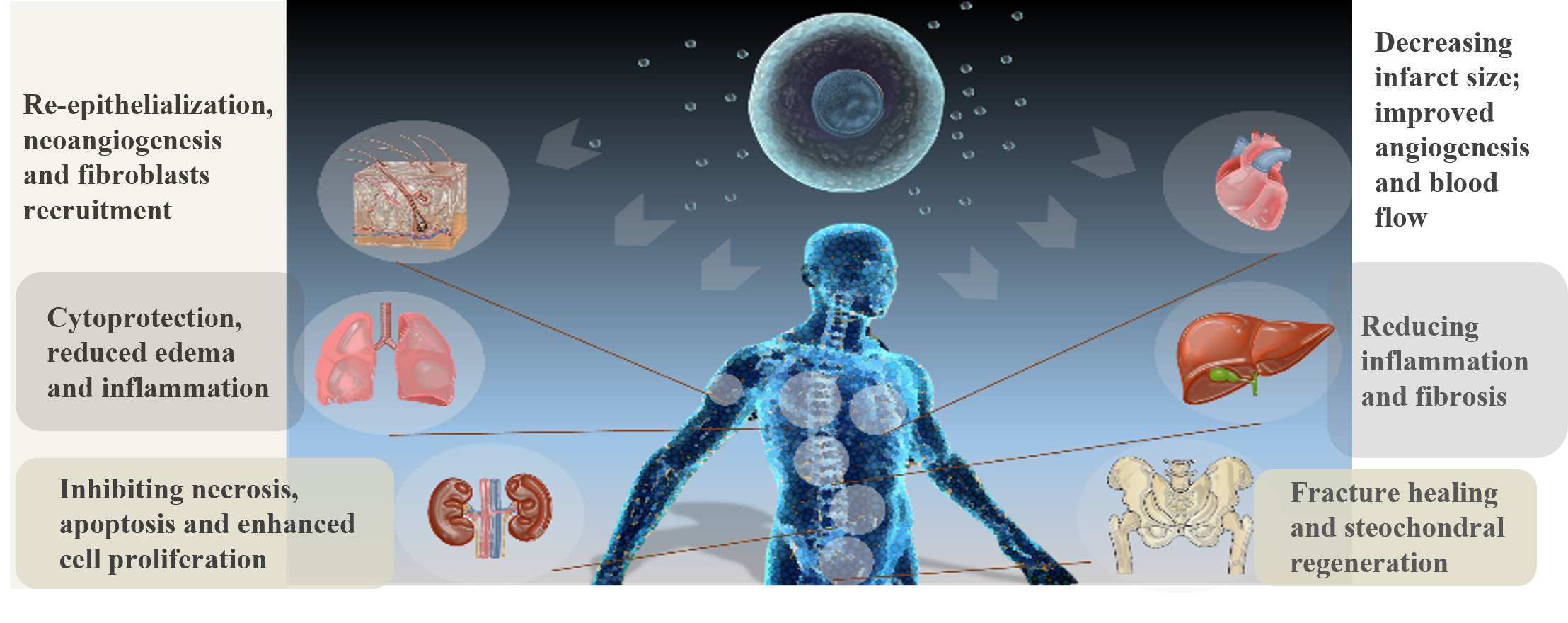
Fig. 1: Regenerative effect of SC EVs may be expected for a multiple organs based on pre-clinical data.
We investigate large-scale high-yield EV production based on our patented concept of turbulence-vesiculation complying with a standardized production in GMP bioreactors in line with regulatory issues. Our set-up relies on the generation of a controlled turbulent flow in which turbulence microvortices will elicit a shear stress on cells triggering EV release (Figure 2). Our approach is bio-inspired based on EV release by the turbulent flow in bloodstream. This turbulence-based strategy is (i) time-saving enabling massive EV release in some hours, (ii) integrated as it is based on tuning the own GMP bioreactor stirring system, (iii) straightforward as no further processing is required to eliminate the trigger (turbulence disappearing when stirring is turned off) and (iv) scalable based on turbulence physical laws.
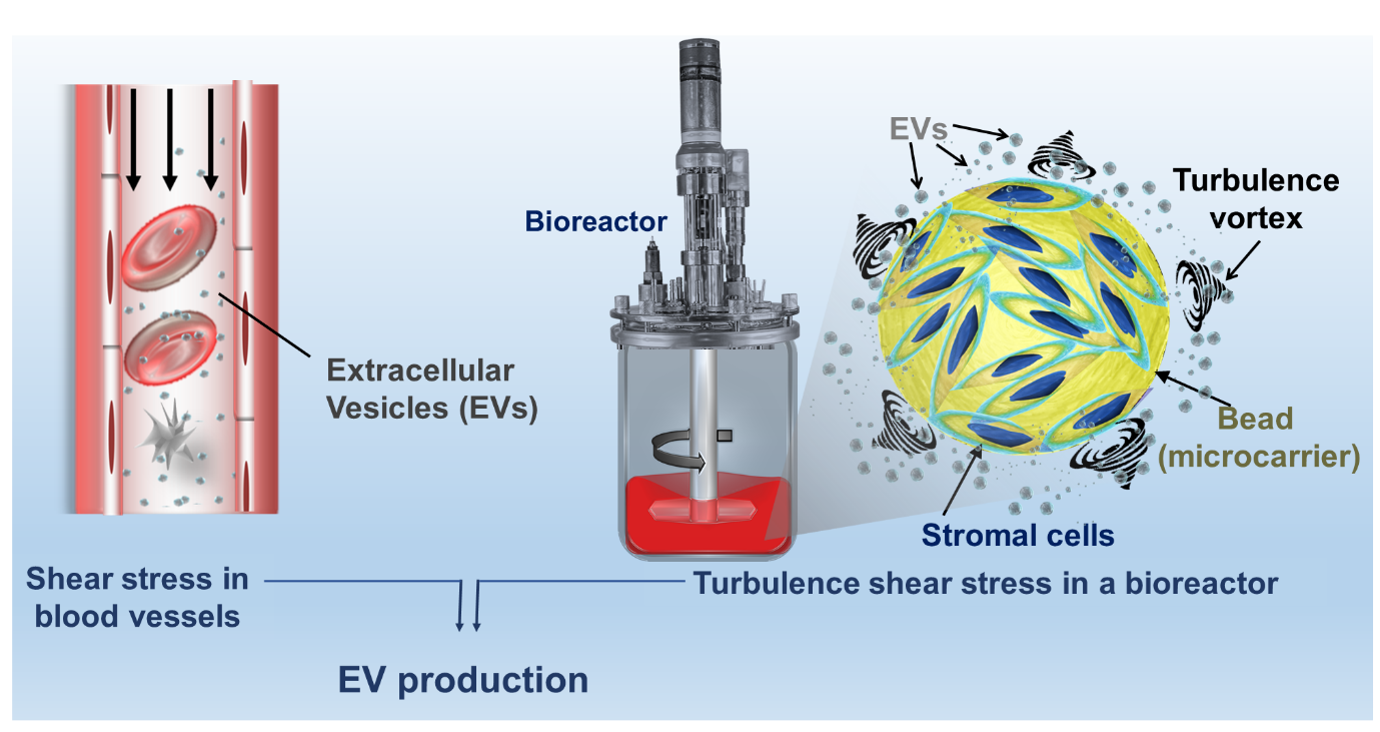
Figure 2: Bio-inspired EV production by a turbulent flow in a bioreactor containing microcarrier-anchored cells. EVs are naturally released in our bloodstream in response to shear stress in blood vessels. Stomal cells are sheared by turbulence-generated microvortices triggering EV release in bioreactors.
We investigate a thermo-actuated EV delivery in the fistula tract (Figure 3) for eliciting an enhanced therapeutic effect in situ. Our strategy is expected to avoid systemic administration clearance and overcome difficulties related to local delivery, such as fistula secretions (washing-out the therapeutic agent) and fistula tract inaccessibility (sometimes irregular large defects of several centimeters). Our approach relies on dual biomaterial/EV component for fistula therapy. The thermoresponsive hydrogel biomaterial component is expected to cope with fistula local delivery difficulties promoting an occlusive effect, retaining EVs in the fistula tract and preventing EV wash-out by fistula secretions, while enabling the filling of the entire fistula tract despite its size and irregular morphology. Biomaterial choice was based on material physical and therapeutic properties and considered a clinical translation perspective. Building on strong preliminary results, we intend to investigate the combination of turbulence EVs with a poloxamer 407 hydrogel. We investigate the off-label use of this hydrogel, which was a vessel occlusive medical device authorized in Europe, as an innovative fistula occlusive EV vehicle. This mechanical occlusive effect has an interest by itself in the therapy of digestive fistulas. Healing process is favored by decreasing the flow of secretions from the digestive system to the skin. Therefore, Our strategy relies on a potential synergic effect of SC EVs and the thermoresponsive gel association.
We fully consider key regulatory and manufacturing issues in the project choices to set the basis for implementing the first future clinical trial on SC EVs in a biomaterial for the therapy of digestive fistulas. Noteworthy, our approaches may be extended to a multitude of EV parent cell types, or therapy indication. Therefore, the advances to be achieved in this project may also be relevant for unmet needs related to other diseases. We hope that our strategies may contribute in the future to democratize EVs as biotherapies for the management of digestive fistulas and other diseases with high morbidity or mortality. In a long-term perspective, by facilitating patient access to last generation therapies, Our technologies may improve the quality of life of refractory patients, tackling medical and societal challenges.
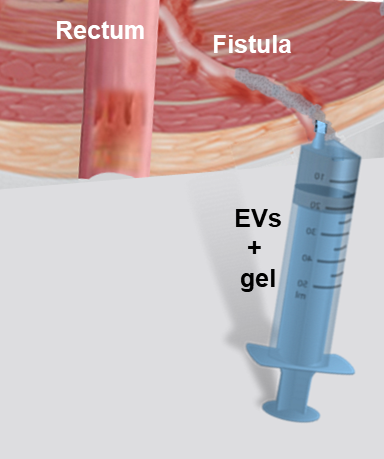
Figure 3: Digestive fistula thererapy by extracellular vesicles into a thermo-responsive gel delivered locally at the digestive fistula.
Enhancing digestive fistula healing by the off-label use of a thermoresponsive vessel occluder polymer associated with esophageal stent placement: A case report. Berger A, Eric Caudron, Perrod G, Boucenna I, Gazeau F, Wilhelm C, Berger A, Clément O, Cellier C, Silva AKA†, Rahmi G†. Clinics and Research in Hepatology and Gastroenterology. 2021, 45 (4) 101474.
Regenerative medicine for digestive fistulae therapy: Benefits, challenges and promises of stem/stromal cells and emergent perspectives via their extracellular vesicles. Sebbagh AC, Rosenbaum B, Péré G, Alric H, Berger A, Wilhelm C, Gazeau F, Mathieu N, Rahmi G, Silva AKA*. Advanced Drug Delivery Reviews. 2021, 113841.
Extracellular vesicles from adipose stromal cells combined with a thermoresponsive hydrogel prevent esophageal stricture after extensive endoscopic submucosal dissection in a porcine model. Coffin E, Grangier A, Perrod G, Piffoux M, Marangon I, Boucenna I, Berger A, M’Harzi L, Assouline J, Lecomte T, Chipont A, Guérin C, Gazeau F, Wilhelm C, Cellier C, Clément O, Silva AKA*†, Rahmi G†. Nanoscale. 2021, 13, 14866-14878.
Local administration of stem cell-derived extracellular vesicles in a thermoresponsive hydrogel promotes a pro-healing effect in a rat model of colo-cutaneous post-surgical fistula. Berger A, Araújo-Filho I, Piffoux M, Nicolás-Boluda A, Grangier A, Boucenna A, Cristiano Real C, Marques FLN, de Paula Faria D, do Rego ACM, Broudin C, Gazeau F, Wilhelm C, Clément O, Cellier C, Buchpiguel CA, Rahmi G†, Silva AKA*†. Nanoscale. 2021, 13, 218-232.
Thermoresponsive Gel Embedding Adipose Stem Cell-Derived Extracellular Vesicles Promotes Esophageal Fistula Healing in a Thermo-Actuated Delivery Strategy. Silva AKA*, Perretta S, Perrod G, Pidial L, Lindner V, Carn F, Lemieux S, Alloyeau D, Boucenna I, Menasché P, Dallemagne B, Gazeau F, Wilhelm C, Cellier C, Clément O, Rahmi, G. ACS Nano. 2018, 12(10):9800-9814.
Axe 4: Extracellular Vesicles (EVs): Analytics and Characterization
Extracellular vesicles (EVs) are submicrometric (30 – 1000 nm) biological objects delimited by a membrane and secreted by most of cell types. They are central to the intercellular communication and the process of molecular exchange between intra- and extracellular media. By recapitulating the biological properties of producer cells, EVs have a increasing interest in translationnal research, especially for theranostics, ie therapeutic and / or diagnostic applications.
Indeed, in a physiopathological context, EVs are natural diagnostic probes circulating in lots of biological fluids (blood, saliva, urine, …) and could be accessible through minimally invasive methods.
When secreted by stem cells, EVs displayed regenerative properties similar to producer cells, offering therefore an alternative solution to cell therapy for regenerative medicine. Their biocompatibility is also a great advantage for their use as therapeutic vectors for drug delivery (ex: chemotherapy) with in addition a preferential organostropism for inflammation sites.
In order to design an analytical toolbox for the quality control of therpeutic EVs as well as to design diagnostic tools based on EVs from different biofluids, MSC-Med created a Core Facility called IVETh for the production, engineering and characterization of EVs for diagnosis and personalized therapy.
Physicists, biologists, chemists, pharmacists and clinicians gathered together within the IVETh core facility to offer a panel of expertises around the physical, chemical and biological characterization of EVs for therapeutic or diagnostic purposes. The IVETh core facility regroups state-of-the-art equipments that are distributed over 4 sites (MSC-Med, IPNP, CRI, PARCC).

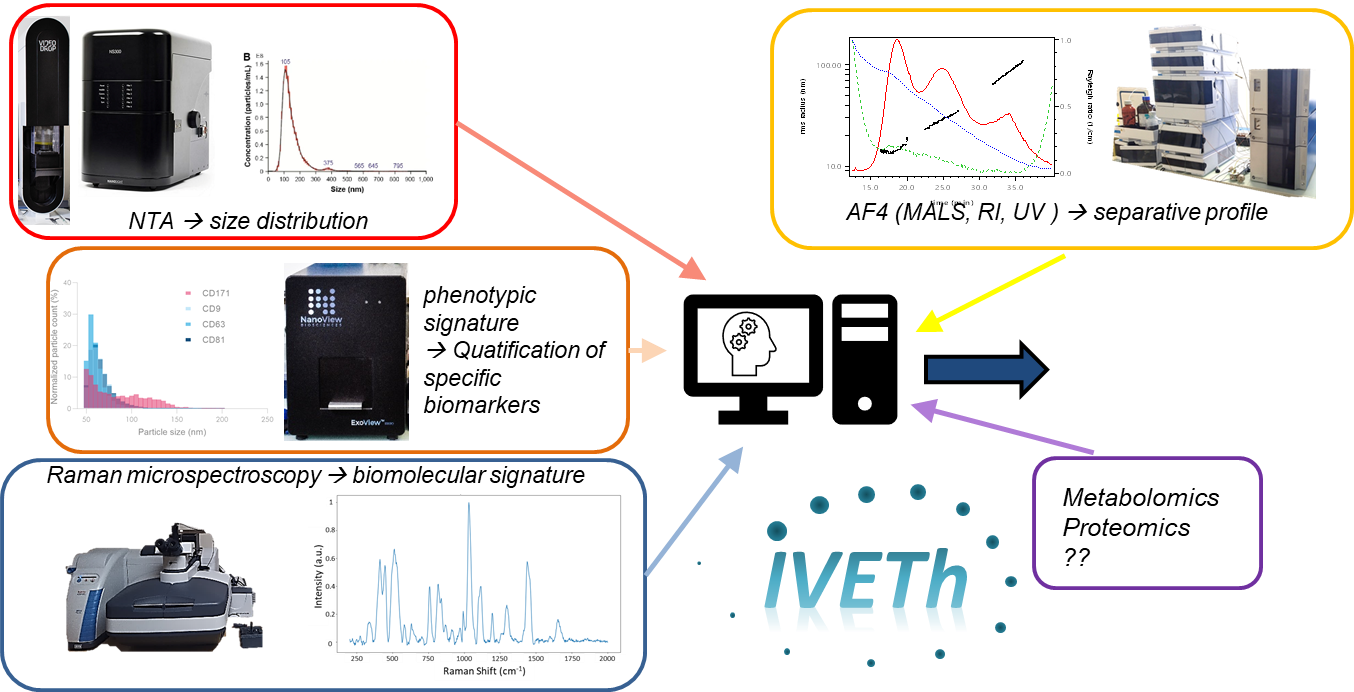
Our facility proposed a panel of cutting edge instruments for characterizing EV size and concentration (based on NTA, ILM), EV biomolecular signature (based on Raman spectroscopy), EV phenotype (based on interferometric detection on immunofunctionnalized substrates), therapeutical potency (based on high content screening & analysis). In addition, the facility also offers access to analytical separation (A4F), purification (UC, TFF) and nanoscopy.

We are also developing statistical methodologies based on machine learning to develop automated classification based on multimodal data.
For more information about our IVETh Core Facility, please visit the dedicated website here.
Axe 5
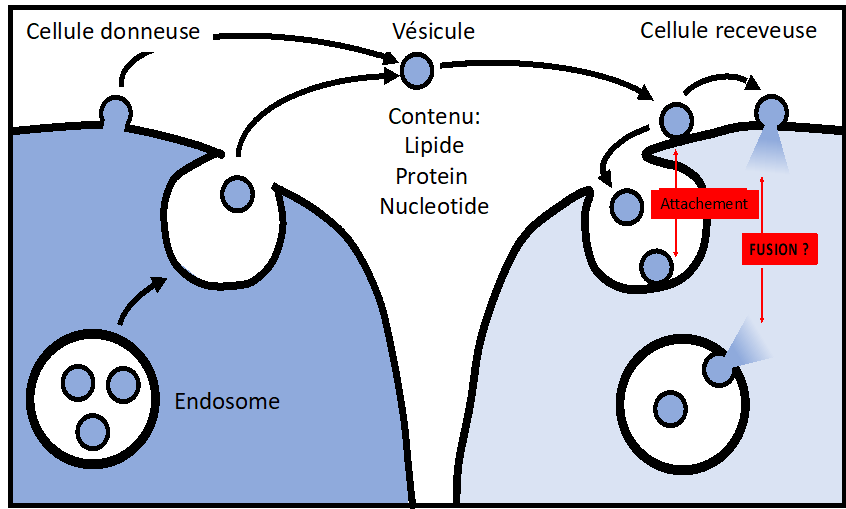
We aim at characterizing the intercellular transport mediated by extracellular vesicles (exosomes), at both cellular and molecular levels. Our goal is to identify the core machinery controlling this process.
Extracellular Vesicles (EVs), including Exosomes, are now recognized as vectors of intercellular communication capable of transferring nucleotides, lipids, and proteins from donor to acceptor cells. EV-mediated communication has been associated with many physiological and pathophysiological functions, including cancer, immune responses, cardiovascular diseases, lipid homeostasis, tissues regeneration and stem cell-based therapy.
EVs are being recognized as vectors of major importance for physiology in general, and appears as promising candidates for translational applications such as targeted therapeutics delivery.
However, the mechanisms responsible for EV delivery within the acceptor cells remain unknown at both the cellular and the molecular levels. How do vesicles enter cells? Is it receptor-dependent? How do vesicles deliver their contents within the cytosol of the acceptor cells? Does it require membrane fusion? If yes, what is the nature of the target membrane and the fusion machinery? Those basic questions are not yet answered.
This is not satisfying, especially considering how much we know about the cellular and molecular mechanisms that regulate the delivery of viruses or the transport of intracellular vesicles, which both share several physico-chemical properties with EVs.
It is therefore of high priority to close these gaps, especially when considering the high translational impact of EVs.
We developed cell-free and cell-based assays to accurately assess in a qualitative and quantitative manner the EV delivery process, especially the EV content release. Our results suggest that EV content delivery occurs within the endo-lysosomal compartment, through a process that is pH- and protein-dependent and that seems to involve membrane fusion. We are now aiming at identifying the core machinery that controls this process, to later engineer EV-mimetics designed for targeted therapeutics delivery.
Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Nat Commun. 2021 Mar 25;12(1):1864. doi: 10.1038/s41467-021-22126-y. PMID: 33767144; PMCID: PMC7994380.
Content release of extracellular vesicles in a cell-free extract. Bonsergent E, Lavieu G. FEBS Lett. 2019 doi: 10.1002/1873-3468.13472.
Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Nat Cell Biol. 2019 doi: 10.1038/s41556-018-0250-9. (revue)
Light-activated cell identification and sorting (LACIS) for selection of edited clones on a nanofluidic device. Mocciaro A, Roth TL, Bennett HM, Soumillon M, Shah A, Hiatt J, Chapman K, Marson A, Lavieu G. Commun Biol. 2018. doi: 10.1038/s42003-018-0034-6.
Spotlight on early-career researchers: an interview with Gregory Lavieu. Commun Biol. 2018. doi: 10.1038/s42003-018-0144-1
Axe 6
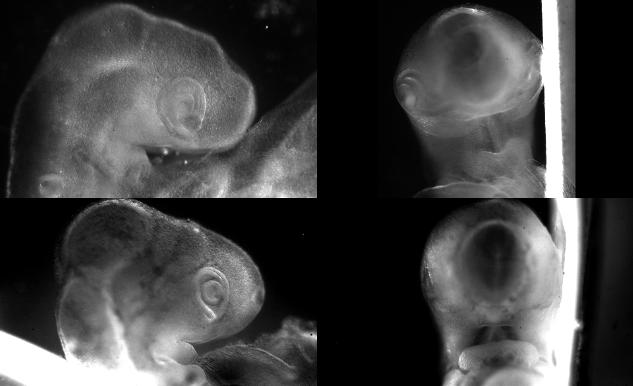
We use embryonic models, especially chicken embryos, but also mouse embryos, to study developmental and regenerative phenomena.
We are interested in biomechanics of development and in biolectricity. We either work in toto with entire embryos, or in organotypic set ups for the culture and study of specific organs. Lately our research topics included :
-Morphogenesis of the early ear pit
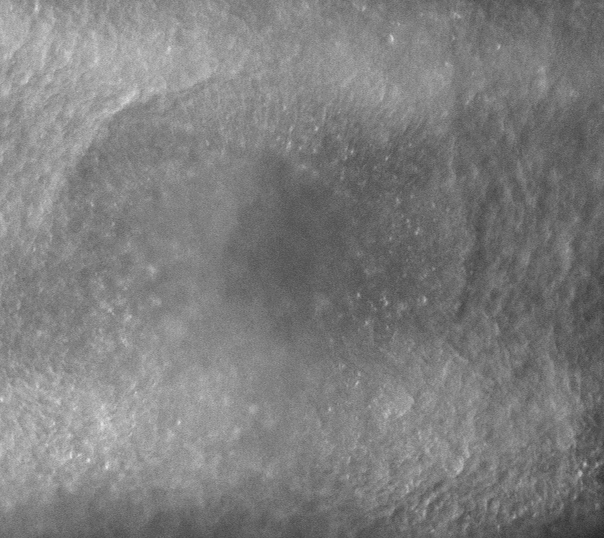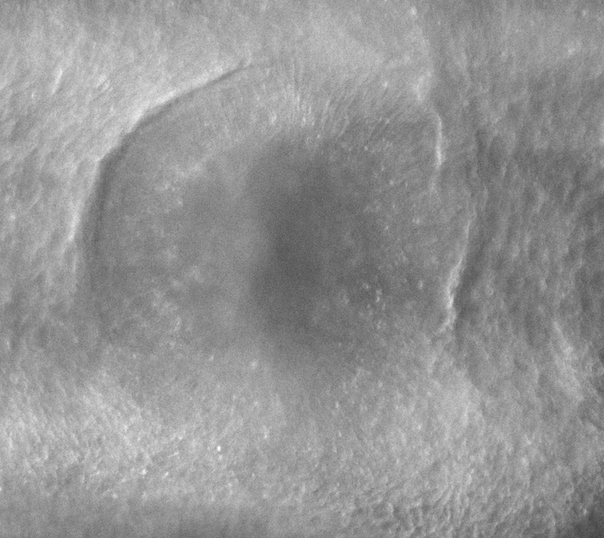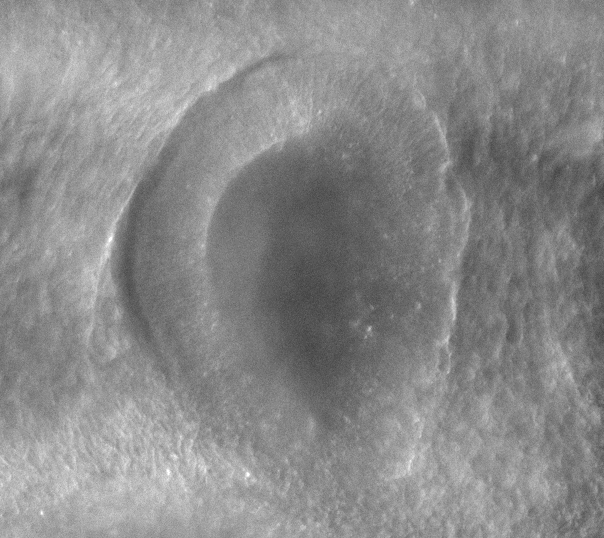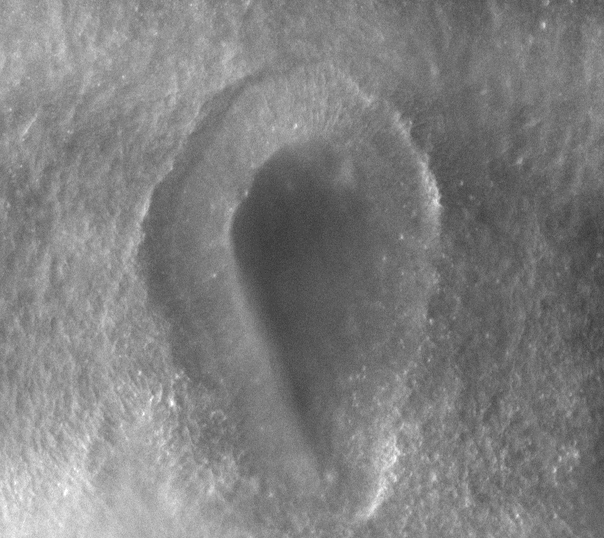
-Morphogenesis of the oro-nasal area
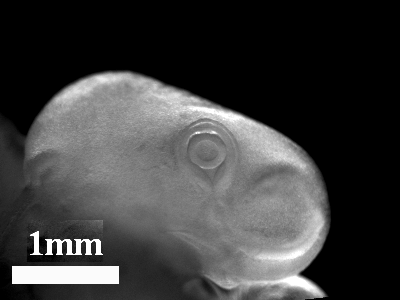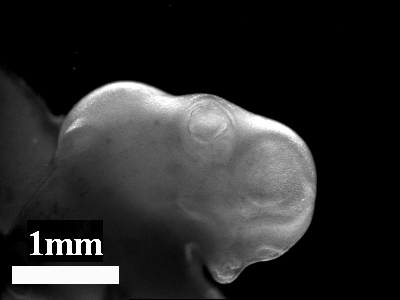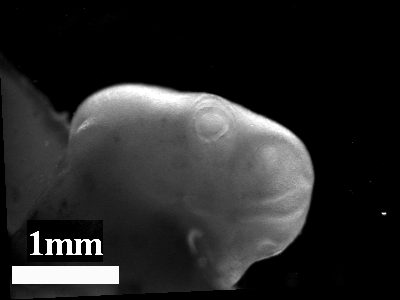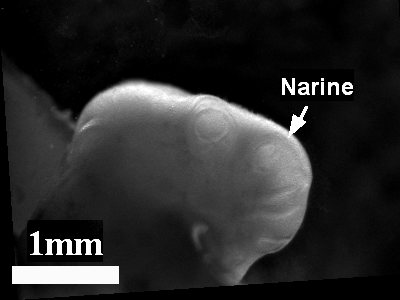
-Eye morphogenesis
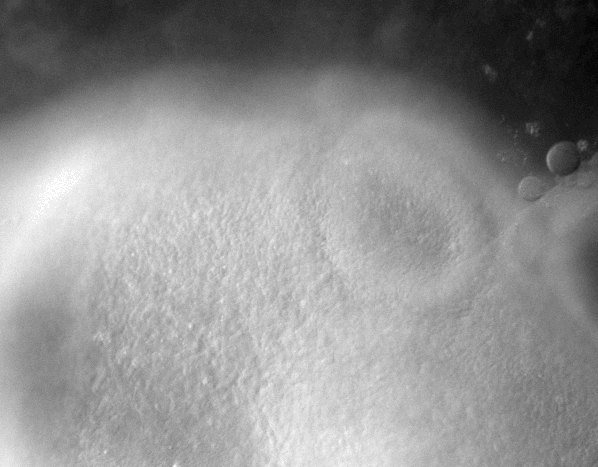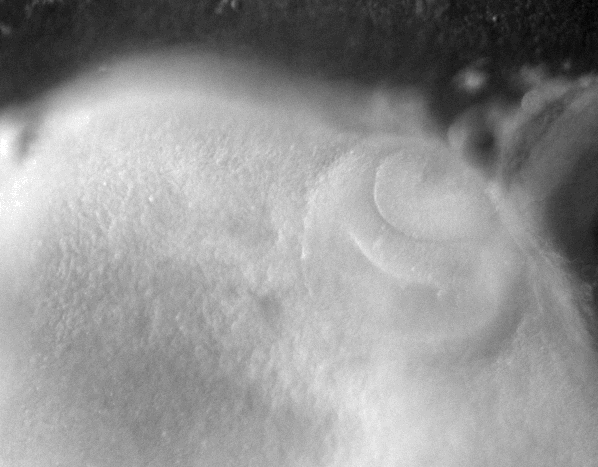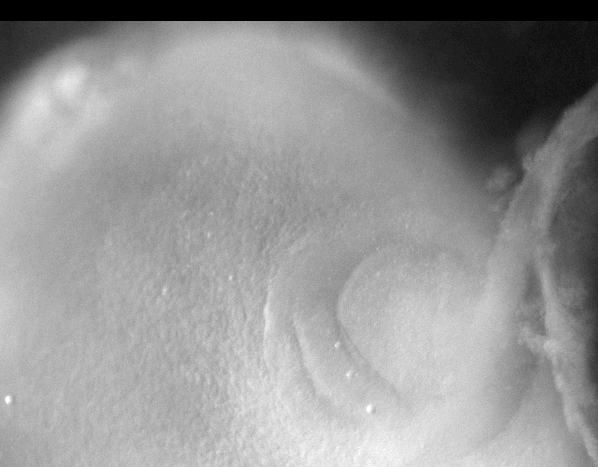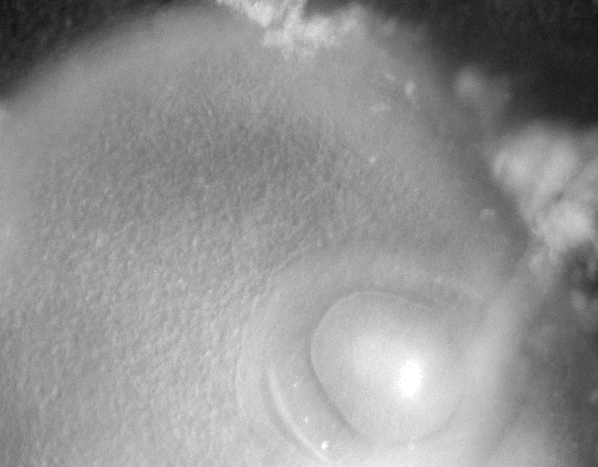
-Electrical stimulation of embryo development
-Vascular morphogenesis in the Chorio-Allantoic Membrane
-Lung regeneration
-Stimulation of limb and jaw growth
-Evolution of craniates
References
2022
Vincent Fleury and Anick Abourachid, A biaxial tensional model for early vertebrate morphogenesis Eu. Phys. J. E 45, 31 (2022).https://link.springer.com/article/10.1140/epje/s10189-022-00184-4
Vincent Fleury, Dynamics of early stages of nose morphogenesis Eu. Phys. J. E 45, 93 (2022).https://link.springer.com/article/10.1140/epje/s10189-022-00245-8
2021
Vincent Fleury, Alexis Peaucelle, Anick Abourachid and Olivia Plateau, Second order division in sectors as a prepattern for sensory organs in vertebrate development, Theory in Biosciences (2021).
2019
Vincent Fleury and Ameya Murukutla, Electrical stimulation of developmental forces reveals the mechanism of limb formation in vertebrate embryos, Eu. Phys. J. E 42, 104 (2019).https://hal.science/hal-02412420
2018
Richard, S., Brun, A., Tedesco, A. , Gallois, B., Taghi, N., Dantan, P., Seguin, J., Fleury, V., Direct imaging of capillaries reveals the mechanism of arteriovenous interlacing in the chick chorioallantoic membrane, Commun Biol 1, 235 (2018).
https://www.nature.com/articles/s42003-018-0229-x